

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aldo-keto reductase family 1 member A1 (Sus scrofa) | BDBM50009832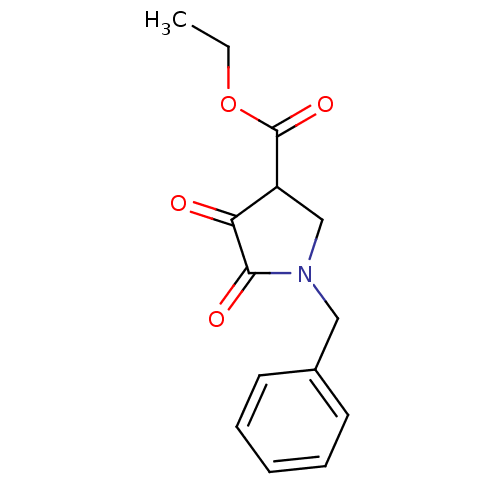 (1-Benzyl-4-hydroxy-5-oxo-2,5-dihydro-1H-pyrrole-3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibitory activity was measured against rat lens aldose reductase in the presence of 1 uM compound with D-glucose as substrates | J Med Chem 34: 1011-8 (1991) Checked by Author BindingDB Entry DOI: 10.7270/Q2NK3FMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member A1 (Homo sapiens (Human)) | BDBM50038843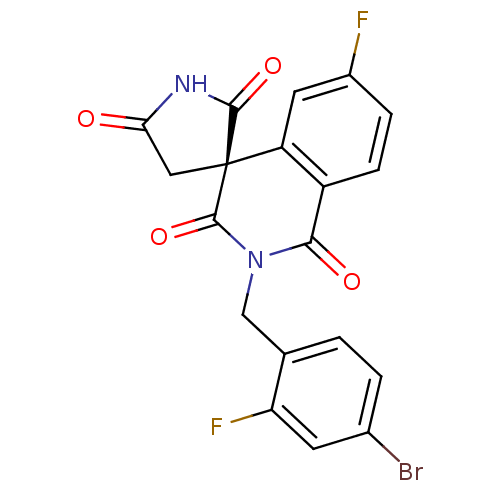 ((4R)-2-(4-bromo-2-fluorobenzyl)-6-fluoro-1H,2'H,5'...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of N-terminal 6His-tagged human aldehyde reductase expressed in Escherichia coli BL21(DE3) mediated D-glucuronate reduction | Bioorg Med Chem 18: 2485-90 (2010) Article DOI: 10.1016/j.bmc.2010.02.050 BindingDB Entry DOI: 10.7270/Q2K35TTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member A1 (Sus scrofa) | BDBM50009844 (2-fluorospiro[9H-fluorene-9,4'-(tetrahydro-1'H-imi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Selectivity ratio measured as the IC50 ratio of aldehyde/aldose reductase values | J Med Chem 34: 1011-8 (1991) Checked by Author BindingDB Entry DOI: 10.7270/Q2NK3FMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member A1 (Rattus norvegicus) | BDBM50009832 (1-Benzyl-4-hydroxy-5-oxo-2,5-dihydro-1H-pyrrole-3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibitory activity was measured against renal inner medulla aldehyde reductase | J Med Chem 34: 1011-8 (1991) Checked by Author BindingDB Entry DOI: 10.7270/Q2NK3FMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member A1 (Rattus norvegicus) | BDBM16312 ((4S)-6-fluoro-2,3-dihydrospiro[1-benzopyran-4,4'-i...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Pisa Curated by ChEMBL | Assay Description In vitro inhibitory activity against aldehyde reductase 1 (ALR1) from rat kidney. | J Med Chem 46: 1419-28 (2003) Article DOI: 10.1021/jm030762f BindingDB Entry DOI: 10.7270/Q21G0KN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member A1 (Sus scrofa) | BDBM50038843 ((4R)-2-(4-bromo-2-fluorobenzyl)-6-fluoro-1H,2'H,5'...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of pig ALR1 | Bioorg Med Chem 17: 1244-50 (2009) Article DOI: 10.1016/j.bmc.2008.12.024 BindingDB Entry DOI: 10.7270/Q20Z734Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member A1 (Rattus norvegicus) | BDBM50330615 (4-Amino-N-(4-bromo-2-fluorobenzyl)-N-(3,5-difluoro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 323 | n/a | n/a | n/a | n/a | n/a | n/a |
Aristotle University of Thessaloniki Curated by ChEMBL | Assay Description Inhibition of Fischer-344 rat kidney AK1A1 | J Med Chem 53: 7756-66 (2010) Article DOI: 10.1021/jm101008m BindingDB Entry DOI: 10.7270/Q2ZS2WRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member A1 (Bos taurus (Cattle)) | BDBM193860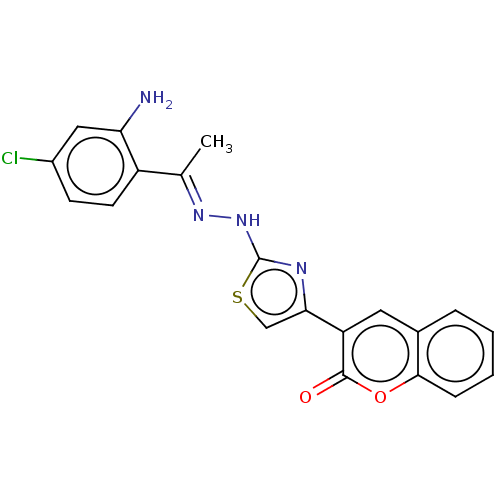 ((E)-3-(2-(2-((2-amino-4-chlorophenyl)(phenyl)methy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 459 | n/a | n/a | n/a | n/a | 6.2 | n/a |
COMSATS Institute of Information Technology | Assay Description The assay results were obtained at 340 nm and ALR1 inhibitory activity was measured with the absorbance change at this respective wavelength. Each we... | Bioorg Chem 68: 177-186 (2016) Article DOI: 10.1016/j.bioorg.2016.08.005 BindingDB Entry DOI: 10.7270/Q2445K96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member A1 (Rattus norvegicus) | BDBM50126777 (CHEMBL33835 | Cyano-[5-fluoro-1-(4-fluoro-benzyl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Pisa Curated by ChEMBL | Assay Description In vitro inhibitory activity against aldehyde reductase 1 (ALR1) from rat kidney. | J Med Chem 46: 1419-28 (2003) Article DOI: 10.1021/jm030762f BindingDB Entry DOI: 10.7270/Q21G0KN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member A1 (Sus scrofa) | BDBM16314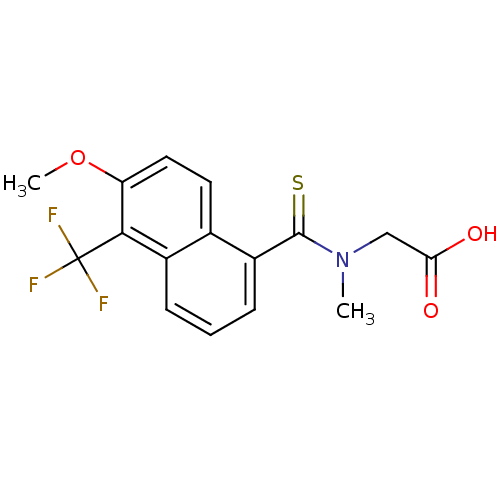 (2-{[6-methoxy-5-(trifluoromethyl)naphthalen-1-yl]-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Selectivity ratio measured as the IC50 ratio of aldehyde/aldose reductase values | J Med Chem 34: 1011-8 (1991) Checked by Author BindingDB Entry DOI: 10.7270/Q2NK3FMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member A1 (Rattus norvegicus) | BDBM50126780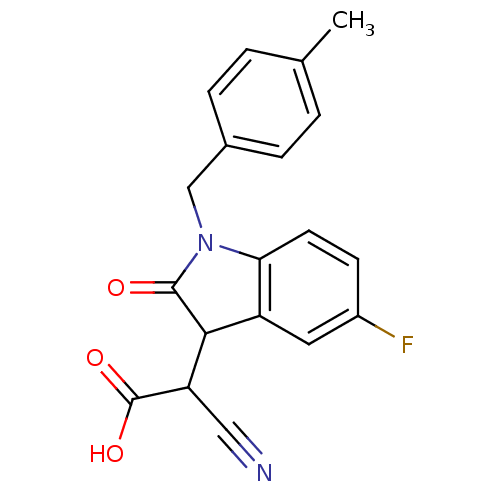 (CHEMBL30993 | Cyano-[5-fluoro-1-(4-methyl-benzyl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Pisa Curated by ChEMBL | Assay Description In vitro inhibitory activity against aldehyde reductase 1 (ALR1) from rat kidney. | J Med Chem 46: 1419-28 (2003) Article DOI: 10.1021/jm030762f BindingDB Entry DOI: 10.7270/Q21G0KN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member A1 (Homo sapiens (Human)) | BDBM16314 (2-{[6-methoxy-5-(trifluoromethyl)naphthalen-1-yl]-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of N-terminal 6His-tagged human aldehyde reductase expressed in Escherichia coli BL21(DE3) mediated D-glucuronate reduction | Bioorg Med Chem 18: 2485-90 (2010) Article DOI: 10.1016/j.bmc.2010.02.050 BindingDB Entry DOI: 10.7270/Q2K35TTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member A1 (Bos taurus (Cattle)) | BDBM193856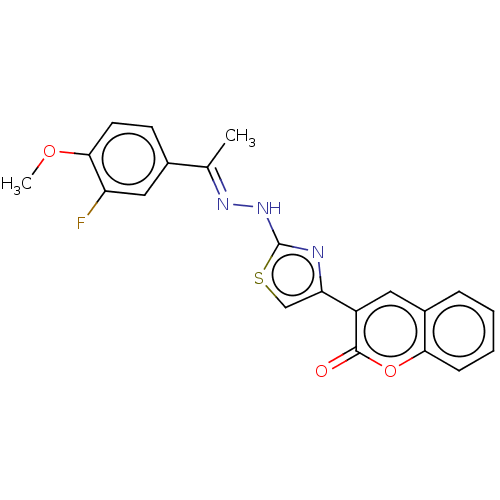 ((E)-3-(2-(2-(1-(3-fluoro-4-methoxyphenyl)ethyliden...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.08E+3 | n/a | n/a | n/a | n/a | 6.2 | n/a |
COMSATS Institute of Information Technology | Assay Description The assay results were obtained at 340 nm and ALR1 inhibitory activity was measured with the absorbance change at this respective wavelength. Each we... | Bioorg Chem 68: 177-186 (2016) Article DOI: 10.1016/j.bioorg.2016.08.005 BindingDB Entry DOI: 10.7270/Q2445K96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member A1 (Homo sapiens (Human)) | BDBM16512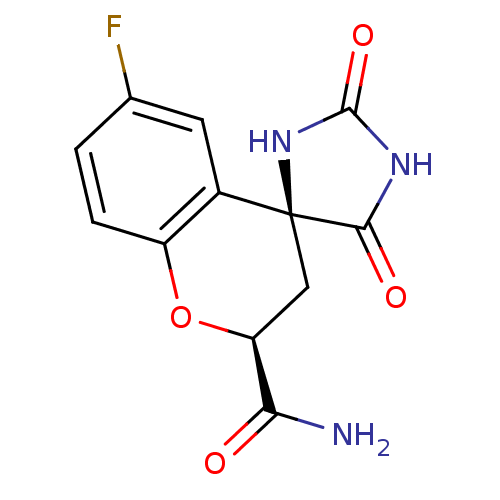 ((2S,4S)-6-fluoro-2',5'-dioxo-2,3-dihydrospiro[1-be...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rational Drug Design Laboratories Curated by ChEMBL | Assay Description Tested for in vitro inhibition activity against human aldehyde reductase (AHR) | J Med Chem 43: 2479-83 (2000) BindingDB Entry DOI: 10.7270/Q2HH6JBT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member A1 (Homo sapiens (Human)) | BDBM16512 ((2S,4S)-6-fluoro-2',5'-dioxo-2,3-dihydrospiro[1-be...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibitory concentration against human ALR1 Aldehyde reductase using DL-glyceraldehyde | J Med Chem 48: 5536-42 (2005) Article DOI: 10.1021/jm050412o BindingDB Entry DOI: 10.7270/Q2K35T6F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member A1 (Rattus norvegicus) | BDBM16314 (2-{[6-methoxy-5-(trifluoromethyl)naphthalen-1-yl]-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibition of Aldehyde reductase 1 | J Med Chem 39: 4396-405 (1996) Article DOI: 10.1021/jm960124f BindingDB Entry DOI: 10.7270/Q2N58N21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member A1 (Bos taurus (Cattle)) | BDBM193858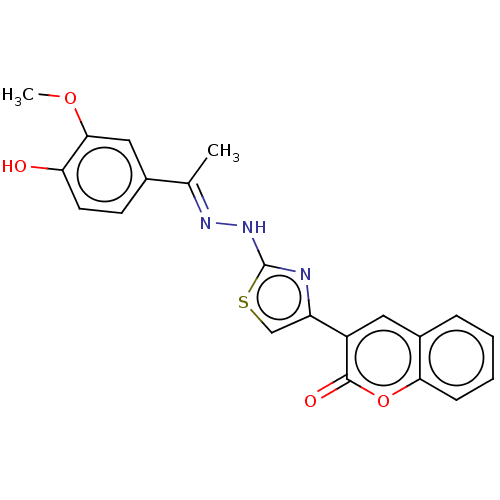 ((E)-3-(2-(2-(1-(4-hydroxy-3-methoxyphenyl)ethylide...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.41E+3 | n/a | n/a | n/a | n/a | 6.2 | n/a |
COMSATS Institute of Information Technology | Assay Description The assay results were obtained at 340 nm and ALR1 inhibitory activity was measured with the absorbance change at this respective wavelength. Each we... | Bioorg Chem 68: 177-186 (2016) Article DOI: 10.1016/j.bioorg.2016.08.005 BindingDB Entry DOI: 10.7270/Q2445K96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member A1 (Homo sapiens (Human)) | BDBM50241817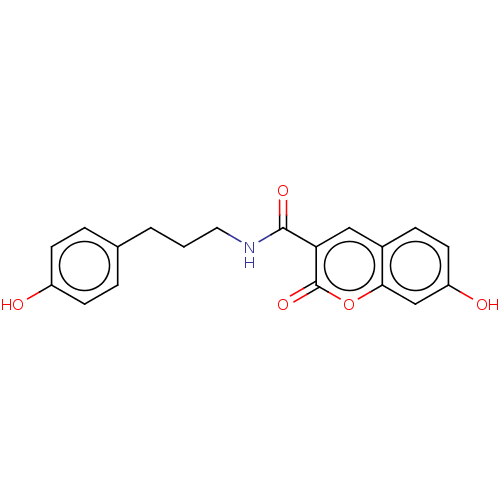 (CHEMBL4081954) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human AKR1A1 using pyridine-3-aldehyde as substrate | J Med Chem 60: 8441-8455 (2017) Article DOI: 10.1021/acs.jmedchem.7b00830 BindingDB Entry DOI: 10.7270/Q2NZ89S7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member A1 (Sus scrofa) | BDBM50049730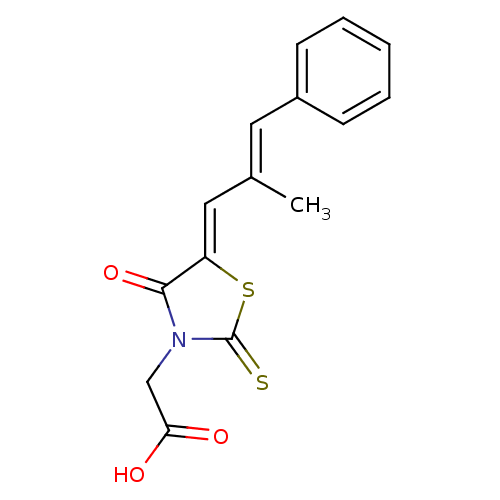 (2-(5-(2-methyl-3-phenylallylidene)-4-oxo-2-thioxot...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Selectivity ratio measured as the IC50 ratio of aldehyde/aldose reductase values | J Med Chem 34: 1011-8 (1991) Checked by Author BindingDB Entry DOI: 10.7270/Q2NK3FMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member A1 (Rattus norvegicus) | BDBM50049730 (2-(5-(2-methyl-3-phenylallylidene)-4-oxo-2-thioxot...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Zoki Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat kidney aldehyde reductase(ALR). | J Med Chem 40: 684-94 (1997) Article DOI: 10.1021/jm960594+ BindingDB Entry DOI: 10.7270/Q2251H84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member A1 (Homo sapiens (Human)) | BDBM50241828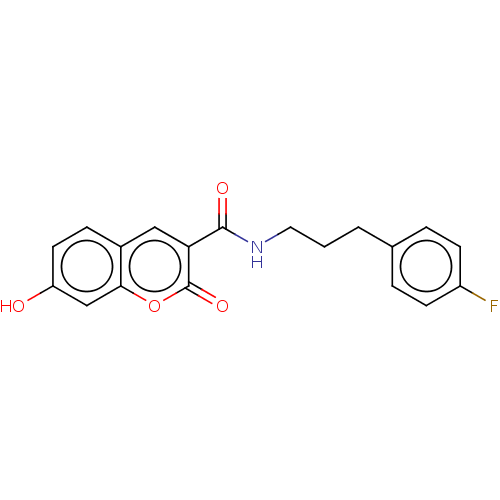 (CHEMBL4089817) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human AKR1A1 using pyridine-3-aldehyde as substrate | J Med Chem 60: 8441-8455 (2017) Article DOI: 10.1021/acs.jmedchem.7b00830 BindingDB Entry DOI: 10.7270/Q2NZ89S7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member A1 (Bos taurus (Cattle)) | BDBM193852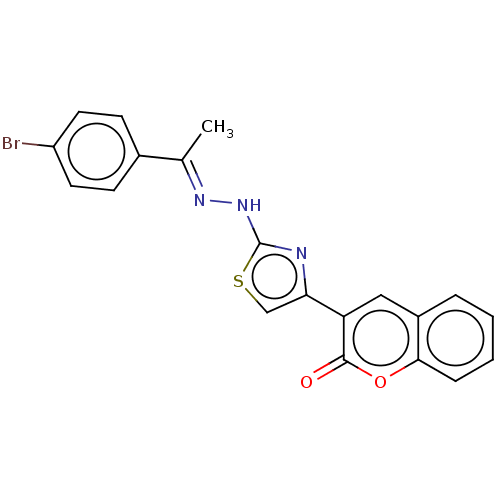 ((E)-3-(2-(2-(1-(4-bromophenyl)ethylidene)hydraziny...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.71E+3 | n/a | n/a | n/a | n/a | 6.2 | n/a |
COMSATS Institute of Information Technology | Assay Description The assay results were obtained at 340 nm and ALR1 inhibitory activity was measured with the absorbance change at this respective wavelength. Each we... | Bioorg Chem 68: 177-186 (2016) Article DOI: 10.1016/j.bioorg.2016.08.005 BindingDB Entry DOI: 10.7270/Q2445K96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member A1 (Rattus norvegicus) | BDBM16312 ((4S)-6-fluoro-2,3-dihydrospiro[1-benzopyran-4,4'-i...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibition of Aldehyde reductase 1 | J Med Chem 39: 4396-405 (1996) Article DOI: 10.1021/jm960124f BindingDB Entry DOI: 10.7270/Q2N58N21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member A1 (Sus scrofa) | BDBM16312 ((4S)-6-fluoro-2,3-dihydrospiro[1-benzopyran-4,4'-i...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibitory activity measured against pig kidney aldehyde reductase using 3-pyridinecarboxaldehyde as substrate | J Med Chem 34: 1011-8 (1991) Checked by Author BindingDB Entry DOI: 10.7270/Q2NK3FMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member A1 (Homo sapiens (Human)) | BDBM16314 (2-{[6-methoxy-5-(trifluoromethyl)naphthalen-1-yl]-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member A1 (Sus scrofa) | BDBM50009777 ((ponalrestat)[3-(4-Bromo-2-fluoro-benzyl)-4-oxo-3,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Selectivity ratio measured as the IC50 ratio of aldehyde/aldose reductase values | J Med Chem 34: 1011-8 (1991) Checked by Author BindingDB Entry DOI: 10.7270/Q2NK3FMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member A1 (Homo sapiens (Human)) | BDBM16462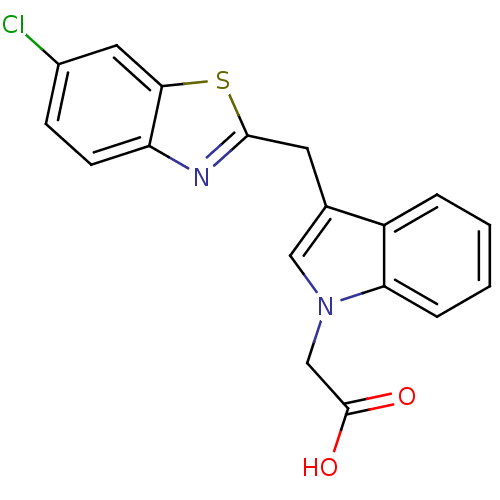 (2-{3-[(6-chloro-1,3-benzothiazol-2-yl)methyl]-1H-i...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member A1 (Sus scrofa) | BDBM16314 (2-{[6-methoxy-5-(trifluoromethyl)naphthalen-1-yl]-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 2.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of pig ALR1 | Bioorg Med Chem 17: 1244-50 (2009) Article DOI: 10.1016/j.bmc.2008.12.024 BindingDB Entry DOI: 10.7270/Q20Z734Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member A1 (Rattus norvegicus) | BDBM7460 (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Pisa Curated by ChEMBL | Assay Description In vitro inhibitory activity against aldehyde reductase 1 (ALR1) from rat kidney. | J Med Chem 46: 1419-28 (2003) Article DOI: 10.1021/jm030762f BindingDB Entry DOI: 10.7270/Q21G0KN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member A1 (Sus scrofa) | BDBM16512 ((2S,4S)-6-fluoro-2',5'-dioxo-2,3-dihydrospiro[1-be...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibitory concentration against porcine ALR1 Aldehyde reductase using DL-glyceraldehyde | J Med Chem 48: 5536-42 (2005) Article DOI: 10.1021/jm050412o BindingDB Entry DOI: 10.7270/Q2K35T6F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member A1 (Homo sapiens (Human)) | BDBM50049730 (2-(5-(2-methyl-3-phenylallylidene)-4-oxo-2-thioxot...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1A1 expressed in Escherichia coli BL21 cells using D-glucuronate as substrate by spectrophotometry | Bioorg Med Chem 20: 356-67 (2011) Article DOI: 10.1016/j.bmc.2011.10.073 BindingDB Entry DOI: 10.7270/Q2N58MTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member A1 (Homo sapiens (Human)) | BDBM50049730 (2-(5-(2-methyl-3-phenylallylidene)-4-oxo-2-thioxot...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of N-terminal 6His-tagged human aldehyde reductase expressed in Escherichia coli BL21(DE3) mediated D-glucuronate reduction | Bioorg Med Chem 18: 2485-90 (2010) Article DOI: 10.1016/j.bmc.2010.02.050 BindingDB Entry DOI: 10.7270/Q2K35TTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member A1 (Homo sapiens (Human)) | BDBM16452 ((4-oxo-3-{[5-(trifluoromethyl)-1,3-benzothiazol-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of N-terminal 6His-tagged human aldehyde reductase expressed in Escherichia coli BL21(DE3) mediated D-glucuronate reduction | Bioorg Med Chem 18: 2485-90 (2010) Article DOI: 10.1016/j.bmc.2010.02.050 BindingDB Entry DOI: 10.7270/Q2K35TTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member A1 (Homo sapiens (Human)) | BDBM16483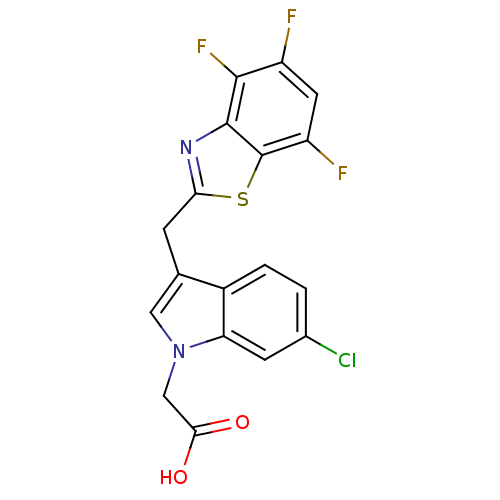 (2-{6-chloro-3-[(4,5,7-trifluoro-1,3-benzothiazol-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member A1 (Bos taurus (Cattle)) | BDBM193848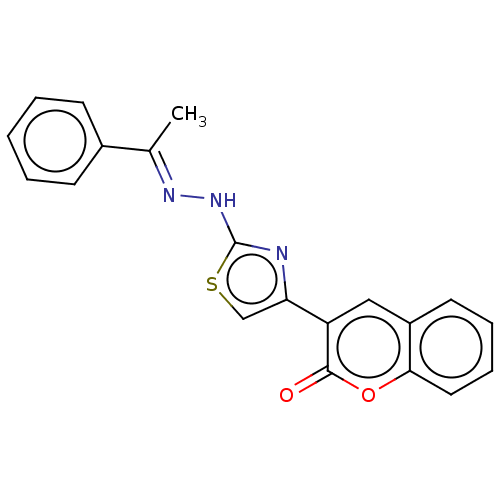 (Coumarin-thiazole series I, 6a) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.94E+3 | n/a | n/a | n/a | n/a | 6.2 | n/a |
COMSATS Institute of Information Technology | Assay Description The assay results were obtained at 340 nm and ALR1 inhibitory activity was measured with the absorbance change at this respective wavelength. Each we... | Bioorg Chem 68: 177-186 (2016) Article DOI: 10.1016/j.bioorg.2016.08.005 BindingDB Entry DOI: 10.7270/Q2445K96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member A1 (Bos taurus (Cattle)) | BDBM193864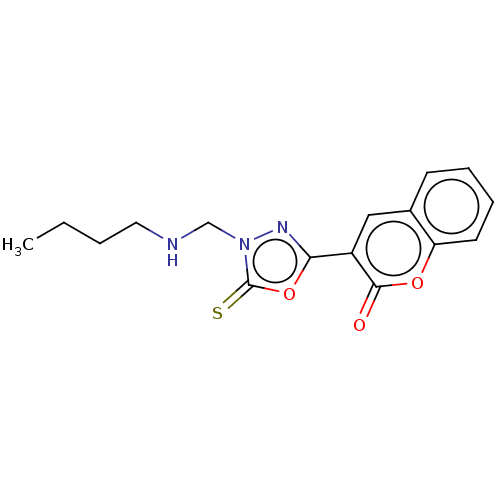 (3-(4-((Butylamino)methyl)-5-thioxo-4,5-dihydro-1,3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.36E+3 | n/a | n/a | n/a | n/a | 6.2 | n/a |
COMSATS Institute of Information Technology | Assay Description The assay results were obtained at 340 nm and ALR1 inhibitory activity was measured with the absorbance change at this respective wavelength. Each we... | Bioorg Chem 68: 177-186 (2016) Article DOI: 10.1016/j.bioorg.2016.08.005 BindingDB Entry DOI: 10.7270/Q2445K96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member A1 (Homo sapiens (Human)) | BDBM16461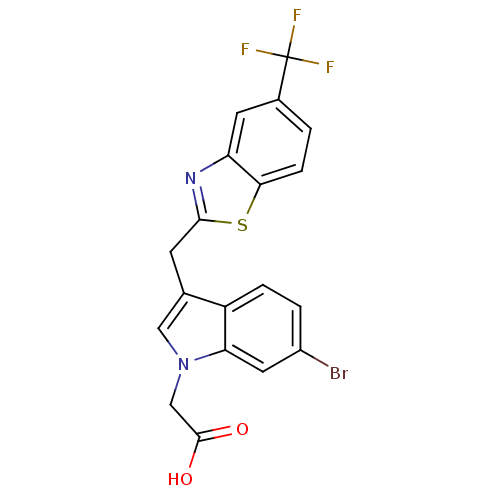 (2-(6-bromo-3-{[5-(trifluoromethyl)-1,3-benzothiazo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member A1 (Rattus norvegicus) | BDBM50126791 (CHEMBL281442 | Cyano-[1-(4-methyl-benzyl)-2-oxo-2,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Pisa Curated by ChEMBL | Assay Description In vitro inhibitory activity against aldehyde reductase 1 (ALR1) from rat kidney. | J Med Chem 46: 1419-28 (2003) Article DOI: 10.1021/jm030762f BindingDB Entry DOI: 10.7270/Q21G0KN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member A1 (Sus scrofa) | BDBM50009832 (1-Benzyl-4-hydroxy-5-oxo-2,5-dihydro-1H-pyrrole-3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibitory activity was measured against pig kidney aldehyde reductase in the presence of 1 uM compound with 3-pyridine carboxaldehyde as substrates | J Med Chem 34: 1011-8 (1991) Checked by Author BindingDB Entry DOI: 10.7270/Q2NK3FMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member A1 (Sus scrofa) | BDBM16312 ((4S)-6-fluoro-2,3-dihydrospiro[1-benzopyran-4,4'-i...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibitory concentration against porcine ALR1 Aldehyde reductase using DL-glyceraldehyde | J Med Chem 48: 5536-42 (2005) Article DOI: 10.1021/jm050412o BindingDB Entry DOI: 10.7270/Q2K35T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member A1 (Homo sapiens (Human)) | BDBM16465 (2-{3-[(6-fluoro-1,3-benzothiazol-2-yl)methyl]-1H-i...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member A1 (Homo sapiens (Human)) | BDBM16312 ((4S)-6-fluoro-2,3-dihydrospiro[1-benzopyran-4,4'-i...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of N-terminal 6His-tagged human aldehyde reductase expressed in Escherichia coli BL21(DE3) mediated D-glucuronate reduction | Bioorg Med Chem 18: 2485-90 (2010) Article DOI: 10.1016/j.bmc.2010.02.050 BindingDB Entry DOI: 10.7270/Q2K35TTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member A1 (Homo sapiens (Human)) | BDBM50363067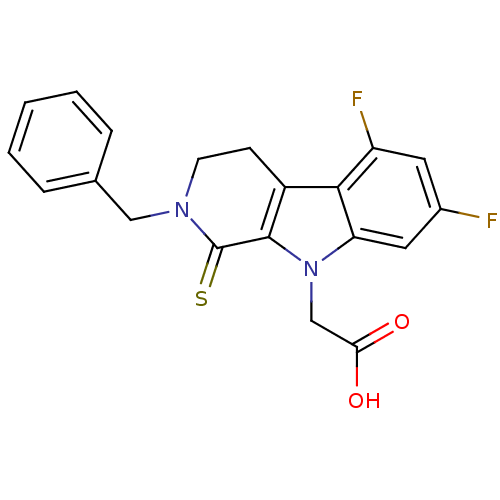 (CHEMBL1944858) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1A1 expressed in Escherichia coli BL21 cells using D-glucuronate as substrate by spectrophotometry | Bioorg Med Chem 20: 356-67 (2011) Article DOI: 10.1016/j.bmc.2011.10.073 BindingDB Entry DOI: 10.7270/Q2N58MTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member A1 (Homo sapiens (Human)) | BDBM16475 (2-{5-bromo-3-[(4,5,7-trifluoro-1,3-benzothiazol-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member A1 (Homo sapiens (Human)) | BDBM16478 (2-[5-(benzyloxy)-3-[(4,5,7-trifluoro-1,3-benzothia...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member A1 (Homo sapiens (Human)) | BDBM16460 (2-(5-chloro-3-{[5-(trifluoromethyl)-1,3-benzothiaz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member A1 (Homo sapiens (Human)) | BDBM16468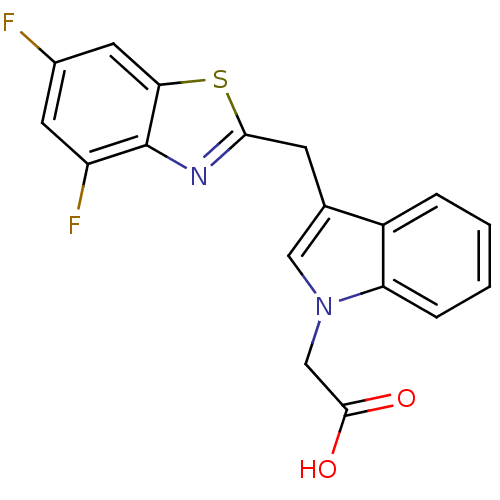 (2-{3-[(4,6-difluoro-1,3-benzothiazol-2-yl)methyl]-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member A1 (Homo sapiens (Human)) | BDBM16312 ((4S)-6-fluoro-2,3-dihydrospiro[1-benzopyran-4,4'-i...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rational Drug Design Laboratories Curated by ChEMBL | Assay Description Tested for in vitro inhibition activity against human aldehyde reductase (AHR) | J Med Chem 43: 2479-83 (2000) BindingDB Entry DOI: 10.7270/Q2HH6JBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member A1 (Homo sapiens (Human)) | BDBM16312 ((4S)-6-fluoro-2,3-dihydrospiro[1-benzopyran-4,4'-i...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibitory concentration against human ALR1 Aldehyde reductase using DL-glyceraldehyde | J Med Chem 48: 5536-42 (2005) Article DOI: 10.1021/jm050412o BindingDB Entry DOI: 10.7270/Q2K35T6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member A1 (Sus scrofa) | BDBM50213307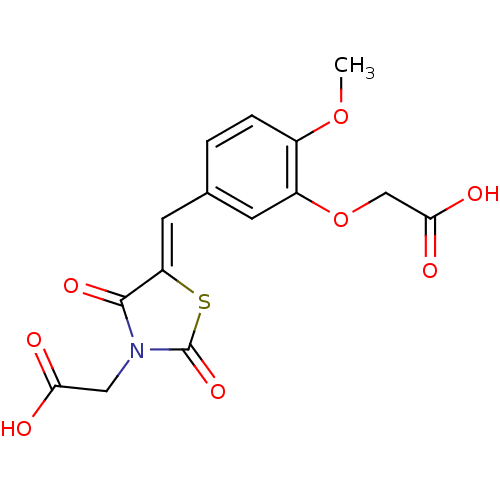 (CHEMBL396176 | [(5Z)-5-{[3-(carboxymethoxy)-4-meth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of pig ALR1 assessed as decrease in NADPH absorbance by spectrophotometry | Eur J Med Chem 45: 1140-5 (2010) Article DOI: 10.1016/j.ejmech.2009.12.019 BindingDB Entry DOI: 10.7270/Q27081KV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 163 total ) | Next | Last >> |